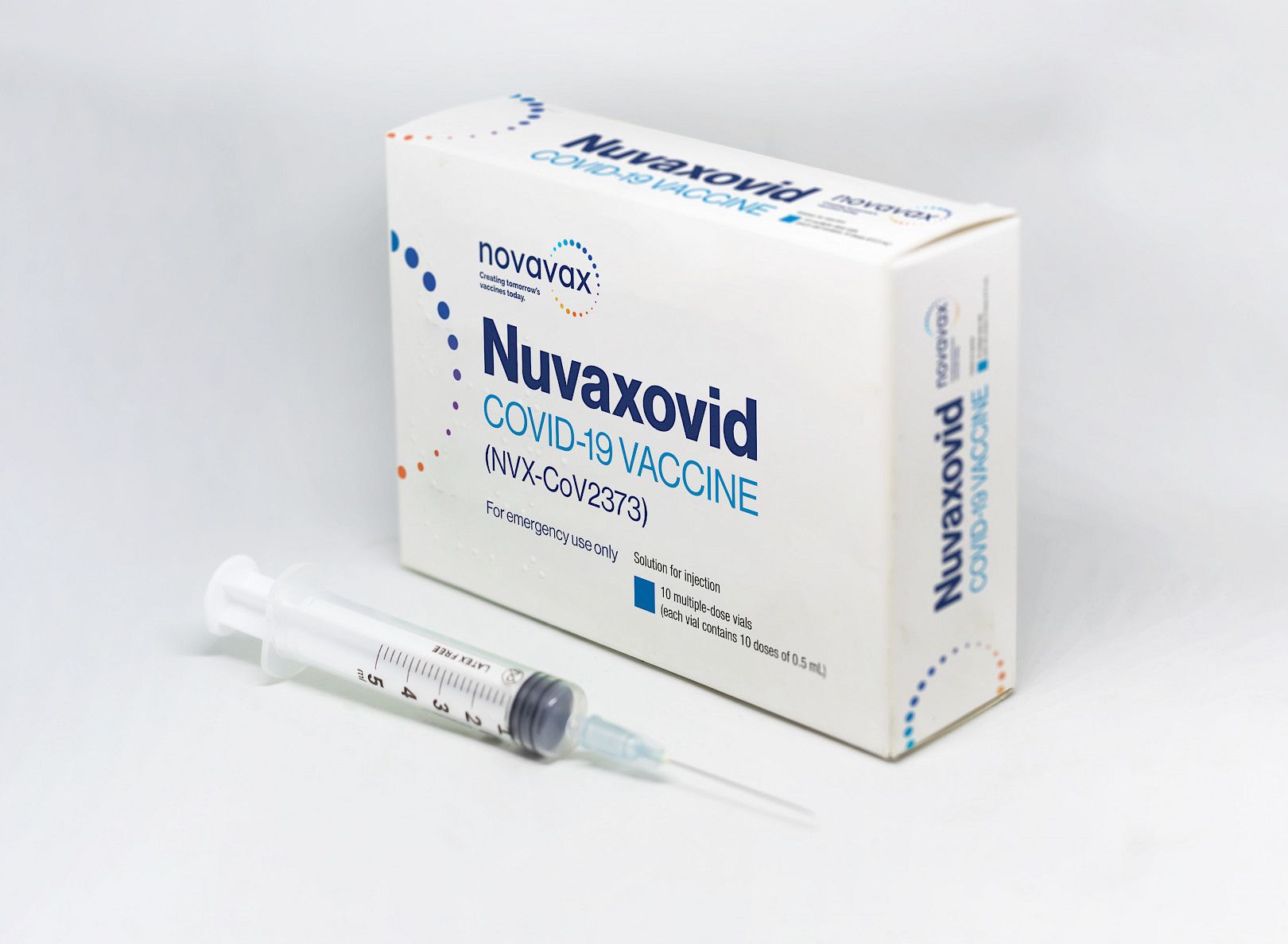
Nuvaxovid
The first batch of Nuvaxovid is expected to arrive in. Nuvaxovid SARS-CoV-2 rS with matrix M adjuvant NVX-CoV2373 was approved for the following therapeutic use.

Coronavaccin Van Novavax Onder Voorwaarden Goedgekeurd Vanaf 18 Jaar Nieuwsbericht College Ter Beoordeling Van Geneesmiddelen
Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022.

. About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach. Active immunisation to prevent coronavirus disease 2019 COVID. Cambridge Mass and Osaka Japan April 19 2022 Takeda today announced that it has received manufacturing and marketing approval from the Japan Ministry of Health.
As at 31 May four cases of adverse reactions were reported out of the 2792 doses of Nuvaxovid administered here at a 014 per cent incidence rate the report published on. Nuvaxovid is the first protein-based COVID-19 vaccine granted. 1 day ago3rd November 2022 0355 GMT11.
Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30. Nuvaxovid contains a version of a protein found on the. This vaccine called Nuvaxovid produced by the company Novavax is a so-called subunit vaccine and differs from the previously approved vaccines.
The addition of the saponin-based. Company Novavax should not be given to individuals. The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix.
Rokotteesta ei myöskään ole haittaa vaikka. Nuvaxovid contains a version of a protein found on the. Nuvaxovid-rokote sopii lähes kaikille aikuisille.
What are subunit vaccines. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. 1 day agoPublicerad idag 0702.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. This CMA has similar requirements to that. The vaccine is safe and effective for all individuals aged 18 and above.
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. 2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US. Nuvaxovid is authorised in Northern Ireland under the CMA granted by the European Medicines Agency on 20 December 2021.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. 1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Det eftersom att data från.
Nuvaxovid is packaged as a ready-to-use liquid formulation in a vial containing 10 doses. The new vaccine was developed by Novavax and the Coalition for Epidemic Preparedness Innovations CEPI and is the originator product for the Covovax TM vaccine. This will enable us to start offering the Nuvaxovid.
Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. Beslutet är temporärt och gäller från. The addition of the saponin-based Matrix-M adjuvant.
Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. In line with the WHO Prioritization Roadmap and the WHO Values Framework older adults health workers.
The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. As of January 2022 approximately 300 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 that causes coronavirus.

Coronavirus Q A On The Nuvaxovid Covid 19 Vaccine Cyprus Mail
Switzerland Approves Novavax S Covid Vaccine For 12 18 Year Olds Swi Swissinfo Ch
New Protein Based Covid 19 Vaccine Could Help Boost Rates Say Pharmacists Cbc News

Slimnica Pieejama Razotaja Novavax Eiropa Razota Vakcina Nuvaxovid Stradina Slimnica
Dossier Coronavirus Sars Cov 2 And Covid 19 Suspected Adverse Reactions Reported In The First Three Months Since Start Of Vaccination With Nuvaxovid Paul Ehrlich Institut
Fda To Authorize Novavax S Covid 19 Vaccine Politico
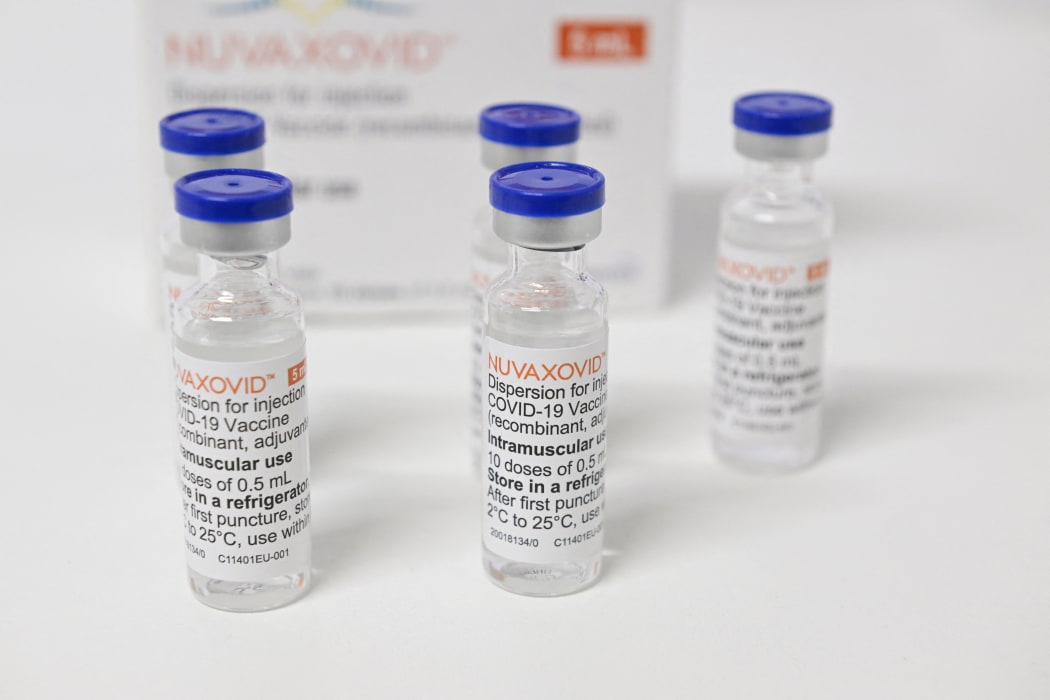
Covid 19 Vaccine Novavax Approved For Use In New Zealand Rnz News
Novavax S Nuvaxovid Covid 19 Vaccine Granted Interim Authorisation In Singapore Cna

Nuvaxovid Fifth Vaccine Against Covid Authorised In Eu Euractiv Com

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary

After Ema Recommendation Novavax S Covid 19 Vaccine Nears Rollout In Europe Fierce Pharma

Vaccine Against Coronavirus Nuvaxovid Novavax Niph

Novavax Hopes Its Covid Shot Wins Over Fda Vaccine Holdouts The Boston Globe

Novavax Covid 19 Vaccine Nuvaxovid Data On Side Effects

Infomesen Long Covid 19 Nuvaxovid Novavax Vaksin Australian Government Department Of Health And Aged Care
Novavax Covid 19 Vaccine Nuvaxovid Provisionally Registered In Australia As A Booster In Individuals Aged 18 And Over Pharmtech Focus
Switzerland Approves Its First Protein Based Covid Vaccine Swi Swissinfo Ch

Ecco Nuvaxovid Il Vaccino Di Novavax E Il Quinto Autorizzato Nell Ue Per Il Sars Cov 2 Sif Magazine